Throughout the years, Cowealth has been proactively engaged in the pursuit of cutting-edge technologies and devices on a global scale. Specifically, in fields such as radiation oncology and ophthalmology, we have successfully introduced a range of internationally renowned medical devices. Furthermore, we deliver departmental training and long-term maintenance services, a commitment that has garnered us numerous accolades. These include multiple prestigious awards such as the Top International Distributor and Outstanding Sales Achievement from various original equipment manufacturers. By introducing such cutting-edge technology, we enable our hospital clients to align more closely with international medical standards and enhance the quality of diagnosis and treatment for complex medical conditions.
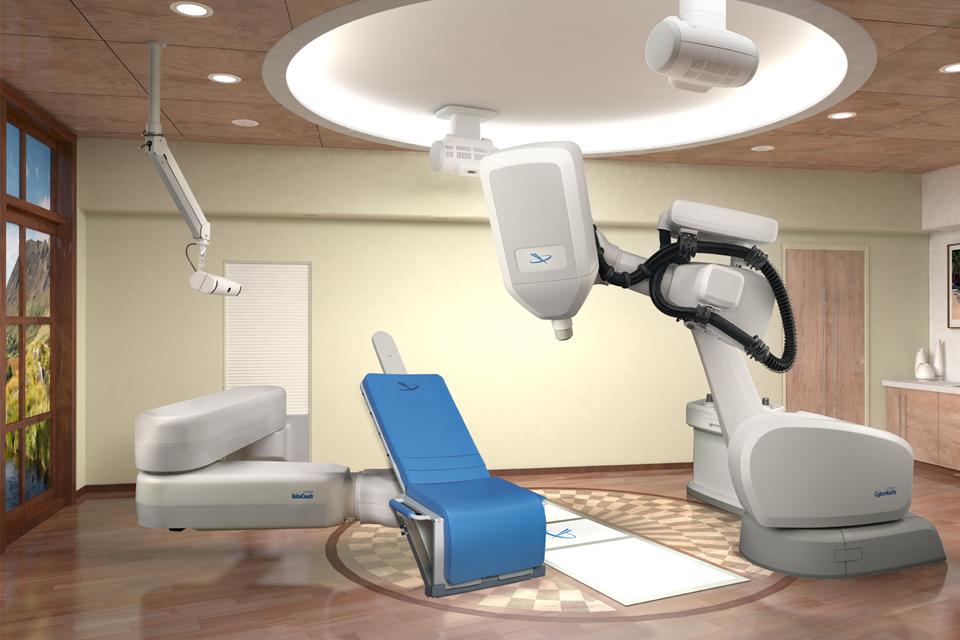
Cowealth provides hospitals with the opportunity to employ the state-of-the-art CyberKnife Stereotactic Radiosurgery System, which is developed by the American company Accuray Incorporated. CyberKnife offers a non-invasive treatment alternative for cancerous and non-cancerous tumors, as well as other medical conditions requiring radiation therapy. With over 20 years of clinical evidence supporting its efficacy, CyberKnife has been used to successfully treat thousands of cancer patients. Moreover, Cowealth presently provides maintenance services for this cutting-edge equipment.
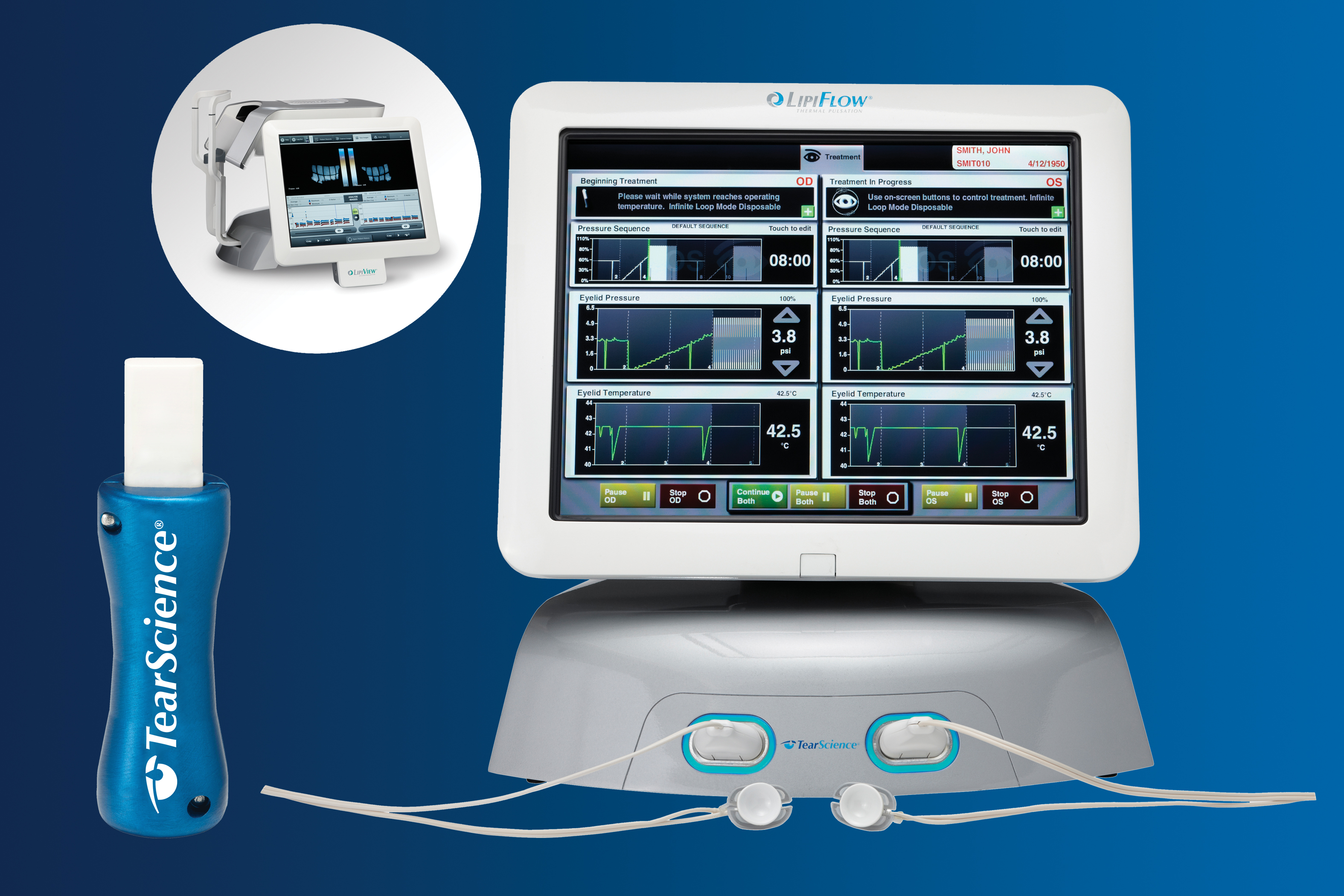
Cowealth represents cutting-edge devices designed to diagnose and address meibomian gland dysfunction (MGD). These include the LipiView Ocular Surface Interferometer and the LipiView Thermal Pulsation System, both developed by the American medical device company Tearscience. LipiView is a highly precise ophthalmic imaging device that relies on unique while light interferometry to observe the lipid layer thickness of a patient’s tear film. The results are used to assess the tear film’s condition. LipiFlow Thermal Pulsation System, an electric thermal pulsation device, is one of the most advanced treatments for meibomian gland dysfunction (MGD).
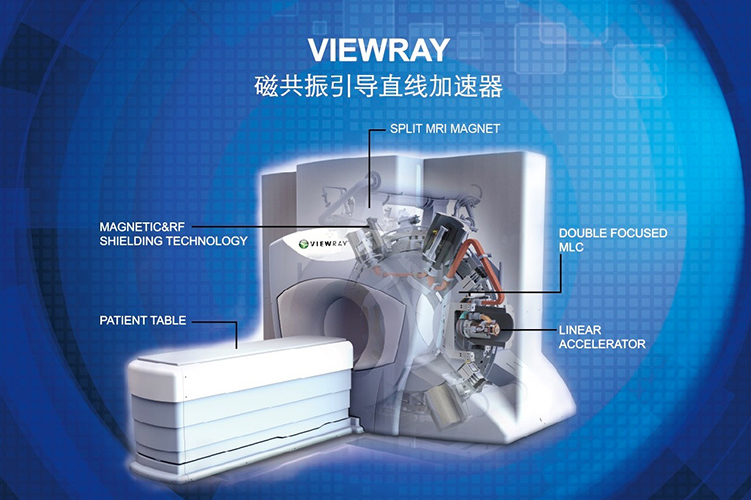
Cowealth introduces the MRIdian-Linac, an MRI-guided radiation therapy system produced by the American company Viewray Incorporated. This innovative technology is the world’s first magnetic resonance-guided linear accelerator certified by the FDA. It combines magnetic resonance imaging and radiation therapy technology to offer high-quality pre-treatment imaging and continuous soft-tissue imaging during treatment. It also supports advanced techniques for radiation therapy. The MRIdian-Linac is capable of targeting tumors with greater precision and delivering high-dose radiation in real time to kill cancer cells, while also safeguarding healthy tissues. This advanced approach offers patients a more personalized and advanced treatment.

Scan
Follow